Principal Investigator
:
Łukasz Suprewicz
Medical University of Bialystok
Panel: NZ6
Funding scheme
: PRELUDIUM 21
announced on
15 march 2022
The SARS-CoV-2 virus, responsible for the outbreak of the COVID-19 pandemic in 2020, has had a huge impact on worldwide healthcare systems. Although it was initially seen as a factor of respiratory infection, it soon turned out it may also lead to severe damage to the central nervous system (CNS). Up to 40% of patients experience chronic neurological symptoms, commonly referred to as "Covid brain fog”. Although some of these (e.g., dizziness) are relatively mild, some patients develop serious sequelae, such as stroke or encephalopathy (brain damage). In light of the ongoing SARS-CoV-2 pandemic, it is crucial to understand the mechanism behind this damage and develop effective treatments.
How SARS-CoV-2 affects the brain remains a subject of intensive research. One hypothesis points to the excessive secretion of inflammatory mediators, known as the “cytokine storm” and the direct action of protein S, which the virus uses to enter host cells. These processes are indicated as some of the factors that damage the barrier between the blood and the structures of the brain (the so-called blood-brain barrier), allowing harmful substances, such as viruses and inflammatory proteins, to pass through in increased quantities.
It is in this context that vimentin makes an appearance. Vimentin is a protein that builds the cytoskeleton of most cells, where it plays a key role in fundamental processes, such as cell division, migration and the protection of the cell nucleus from mechanical damage. When released into the bloodstream by immune cells in response to inflammation, it is known as extracellular vimentin. There are two forms of extracellular vimentin: that which is bound or unbound to the cell surface. In an earlier project, we showed that extracellular vimentin acts as a co-receptor for SARS-CoV-2, which means it is necessary for a cell to be effectively infected (Suprewicz et. al. Small 2022).
 Łukasz Suprewicz, photo by Michał Łepecki
The purpose of this project is to focus on the role of extracellular vimentin in CNS inflammation associated with a SARS-CoV-2 infection. We investigate the impact of extracellular vimentin on different cell types present in the brain, such as endothelial cells and astrocytes, using a 3D blood-brain barrier flow model to assess the permeability and integrity of endothelial cells, and the adhesion and migration of immune-response cells in the presence of vimentin and the SARS-CoV-2 protein. In the next stage, we will evaluate the impact of the substances in question on inflammation by analysing the secretion of inflammatory mediators. The study will culminate in a molecular-level assessment of the signaling pathways characteristic of SARS-CoV-2 infections and inflammatory blood vessel damage.
Łukasz Suprewicz, photo by Michał Łepecki
The purpose of this project is to focus on the role of extracellular vimentin in CNS inflammation associated with a SARS-CoV-2 infection. We investigate the impact of extracellular vimentin on different cell types present in the brain, such as endothelial cells and astrocytes, using a 3D blood-brain barrier flow model to assess the permeability and integrity of endothelial cells, and the adhesion and migration of immune-response cells in the presence of vimentin and the SARS-CoV-2 protein. In the next stage, we will evaluate the impact of the substances in question on inflammation by analysing the secretion of inflammatory mediators. The study will culminate in a molecular-level assessment of the signaling pathways characteristic of SARS-CoV-2 infections and inflammatory blood vessel damage.
The project may significantly increase our knowledge of the effects of extracellular vimentin and its modified forms on endothelial and immune cell responses in a SARS-CoV-2 infection. This, in turn, may contribute to developing more effective treatments for COVID-19 patients, especially those with neurological complications. The project may also identify new therapeutic targets to help fight potential future global pandemics, considering that the role of extracellular vimentin as a co-receptor is not restricted to SARS-CoV-2 but also applies to other infections, including those caused by bacteria.
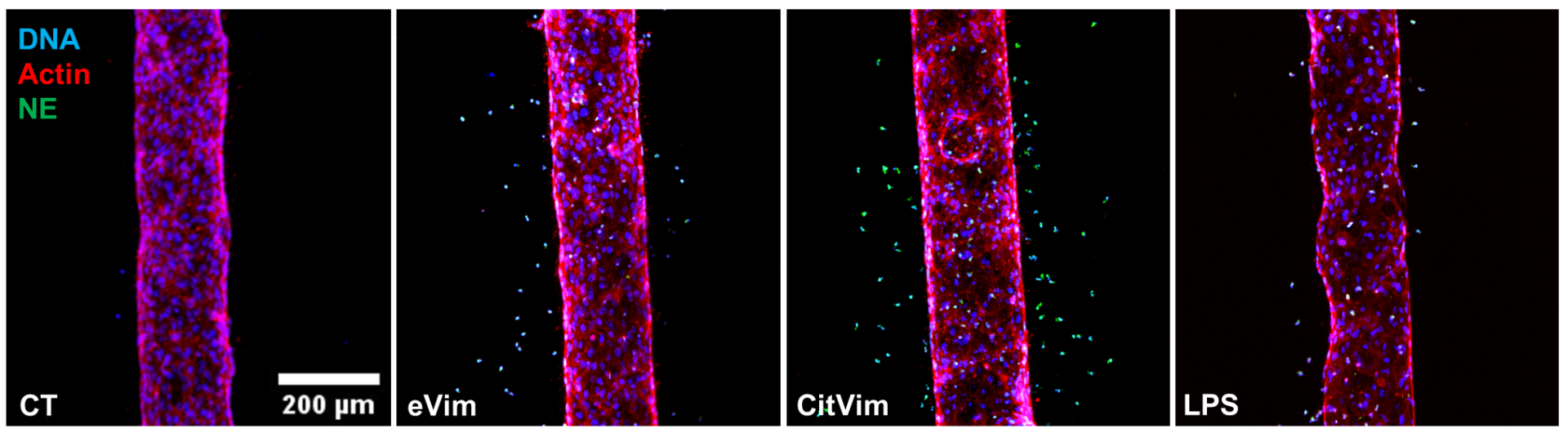
Diapedesis of human neutrophils, untreated or stimulated by vimentin (Vim), citrullinated vimentin (CitVim), or lipopolysaccharide (LPS) in a 3D model of blood vessels. Neutrophil elastase (green), actin (red), DNA (blue)..
Project title: Extracellular vimentin as a signaling molecule in the pathogenesis of inflammation and central nervous system damage in patients with COVID-19
Łukasz Suprewicz
Graduate of the Medical University of Białystok and laboratory diagnostician. PhD student (4th year) at the Doctoral School of the Medical University of Białystok. Author and co-author of over twenty publications in international press/ journals. His research focuses on the molecular mechanisms of interaction between proteins, pathogens and immune-response cells. PI in a PRELUDIUM 21 project and member of a PRELUDIUM BIS1 team, headed by Prof. Dr hab. Robert Bucki.